Food, drugs, cosmetics and medical devices are highly regulated by the Food and Drug Administration (FDA). And they face fierce competition for the attention of consumers. The scrutiny by the FDA and the battle in the marketplace demand courage, imagination and extraordinary technical and legal skills.
Venable attorneys have decades of FDA and marketplace experience. We help clients at every step of the product life cycle—from product development and regulatory approval/clearance to advertising, marketing and distribution.
Protecting your product while it’s still just an idea.
You work hard to develop your products. And we’ll work just as hard to do what’s required to protect them. Trade secrets, patents, trademarks and copyrights are all Venable strong suits. We structure and negotiate licenses, research contracts and technology transfer agreements. And we know how to protect your product and preserve your ability to market and profit from it.
RESOURCES AT EVERY STEP—FROM IDEA TO STORE SHELF
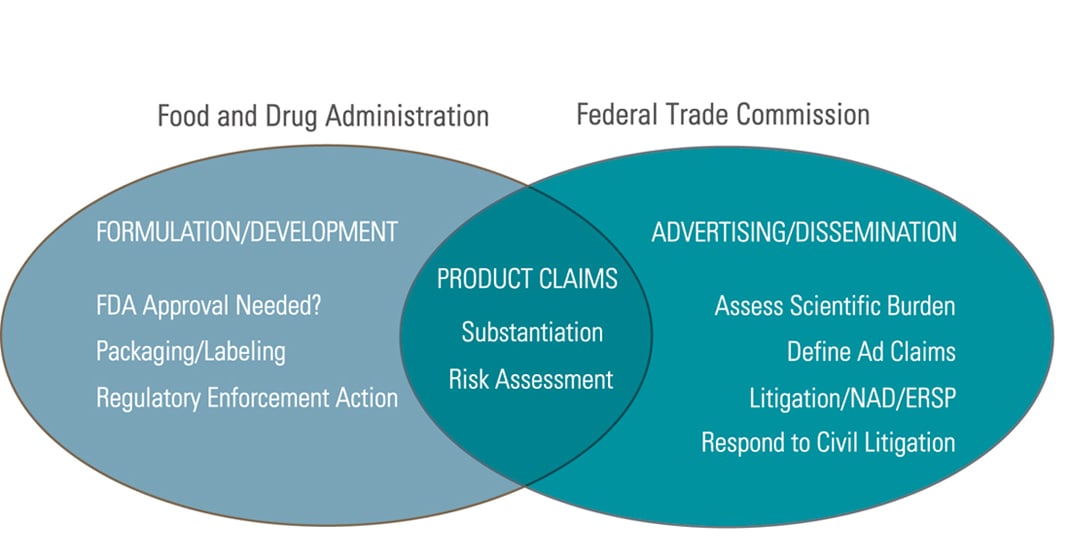
Check those claims before you go to market.
Regulated consumer products constitute a huge and burgeoning market. Dietary supplements and functional foods alone account for more than $15 billion per year in sales. Success often requires aggressive marketing, which can raise legal challenges—starting with the claims you make. Venable attorneys can help you assess the risks your campaign may entail—including claims addressed in the Dietary Supplement Health and Education Act and the Nutrition Labeling and Education Act. We also help assess the proof required for product claims under FTC regulations and what you can say (and cannot say) about competing products. We routinely conduct ingredient reviews and product safety assessments, and evaluate food contact ingredients.
We work closely with Venable’s Marketing and Advertising attorneys to ensure that your message will reach its audience with the impact you desire, without running afoul of regulatory issues.
Help when trouble strikes.
It happens: someone challenges your product or your advertising. It could come from an individual or a class action suit. Or it could be a government agency. We have years of experience in dealing with:
- Adverse Event Reports;
- challenges before the Federal Trade Commission;
- competitor challenges to advertising in proceedings before the National Advertising Division of the Better Business Bureau or the Electronic Retailing Self-Regulation Program;
- current good manufacturing practices;
- false advertising (Lanham Act) litigation;
- food additives;
- food contact substances;
- FDA import detentions;
- FDA inspections;
- FDA warning letters;
- patent, trademark and copyright litigation;
- Proposition 65 litigation;
- state attorneys general; and
- other challenges faced by product developers and manufacturers.
At every step, you’ll work with Venable attorneys whose experience and skills match the issues you face—from gaining FDA approvals to defending you in consumer litigation. You’ll always have the support of a core group of attorneys who understand your business and know how to help you get your products to market.